
Control of an outbreak usually requires identification of the source or reservoir of the virus, followed by cleanup, quarantine, immunization, or a combination of these measures. The first step in controlling an outbreak of gastroenteritis or hepatitis A is identification of the food, water, or possibly the day-care center that is the source of the outbreak. Education programs can promote compliance with immunization programs and help people change lifestyles associated with viral transmission. Such programs have had a significant impact in reducing the prevalence of vaccinepreventable diseases such as smallpox, polio, measles, mumps, and rubella. Bibliography Carter J, Saunders V: Virology: principles and applications, Chichester, England, 2007, Wiley. Websites New Medical Information and Health Information: Antiviral drugs: Antiviral agents, antiviral medications. Food and Drug Administration: Influenza (flu) antiviral drugs and related information. A patient has been exposed to influenza A virus and is in his fifth day of symptoms. To what therapeutic agents is the patient referring, and why did you decline to use the treatment Steps in viral replication that depend on cellular processes are generally poor antiviral drug targets. Amantadine and rimantadine inhibit influenza A virus replication by preventing uncoating of the virus in the cytoplasm. Oseltamivir and zanamivir are neuraminidase inhibitors that inhibit both influenza A and B virus by preventing proper release of the virus. These drugs are effective as prophylactics and before inflammatory and immune responses are generated. It is unlikely that any of these drugs would be effective on the fifth day after symptoms have started. Disinfection requires rigorous treatment for disinfection, such as 2% glutaraldehyde (a protein cross-linking fixative), toilet bowl cleaner with 23% hydrochloric acid and quaternary ammonium compounds, or 10% bleach (sodium hypochlorite). Aerosol spread of influenza A is so contagious that vaccination is the best prevention. Universal precautions include wearing protective clothes, gloves, and eye shields, and not bending, recapping, or removing contaminated needles and other contaminated sharps. A 42-year-old man comes to his physician 9 months after a lung transplant, complaining that he has double vision, difficulty speaking, feels that his muscles do not work right, has difficulty with balance, has tingling of his hands and feet, and keeps forgetting things. A month later, he has difficulty speaking and needs assistance with normal daily functions. He is treated with cidofovir, and his immunosuppressive therapy is eased, but his disease progresses to paralysis and he dies. A biopsy of the brain shows lesions with sites of demyelination, astrocytosis with atypical nuclei, and many histiocytes. Without virus replication to kill the cell and without p53 to errorcheck mutations, there is a greater potential for abnormal, uncontrolled growth of cells, mutation, and development of cervical carcinoma. The virus is transmitted by direct contact (warts) and on fomites (towels) or mixing and matching of mucous membranes (oral and genital papillomas). The virus is harbored in the basal keratinocytes, little virus protein expression occurs, and the infected cells escape recognition. Virions are produced as the cells terminally differentiate and are released from cells that are programmed to die and be shed (skin and mucous epithelium), and therefore, unless vaccinated, little antibody is produced. Immunoglobulin (Ig)A or IgG secreted into the mucosa can prevent infection (if vaccinated) or reinfection of other sites but not other people. Infection of astrocytes results in abnormal growth and appearance; production of virus in oligodendrocytes is lytic and causes demyelination. This includes organ transplant recipients, chemotherapy patients, and interestingly, people who are treated with natalizumab, which blocks the interaction of 4-integrin on immune cells with vascular cell adhesion molecule 1, and this prevents T-cell interactions with antigen-presenting cells and their ability to cross the blood-brain barrier. These viruses are capable of causing lytic, chronic, latent, and transforming infections, depending on the host cell.
Syndromes
T cells with Mls-1aresponsive receptors encoded by V6 are seen in both the cortex and medulla of Mls-1b mice (top panel, cells stained brown with anti-V6 antibody). Note that the mature cells in the medulla express higher levels of the receptor and thus stain more darkly than the immature cells in the cortex. In Mls-1a mice (lower panel) there is no obvious reduction in the number of immature cortical T cells expressing the V6 receptor, but the mature cells are not found. The specificity and strength of signals for negative and positive selection must differ. The answers to these questions are still not known for certain, but two possible mechanisms have been suggested. We will briefly describe each, before going on to discuss some recent experiments that bear on the mechanism of positive selection. First, the interactions that lead to positive selection must include more receptor specificities than those that lead to negative selection. Otherwise, all the cells that were positively selected in the thymic cortex would be eliminated by negative selection, and no T cells would ever leave the thymus. The specificity or affinity of positive selection must differ from that of negative selection. If the specificity and/or avidity of positive and negative selection were the same (left panels), all the T cells that survive positive selection would be deleted during negative selection. Only if the specificity and/or avidity of negative selection is different from that of positive selection (right panels) can thymocytes mature into T cells. Two main hypotheses have been proposed to account for these differences between positive and negative selection. Thymocytes that are signaled weakly are rescued from apoptosis and are thus positively selected, whereas thymocytes that are signaled strongly are driven to apoptosis and are thus negatively selected. Because more complexes are likely to bind weakly rather than strongly, this will result in the positive selection of a larger repertoire of cells than are negatively selected. Alternatively, the delivery of incomplete activating signals by self peptides could account for positive selection: we will call this the differential signaling hypothesis. Under this hypothesis, it is the nature of the signal delivered by the receptor, not just the number of receptors engaged, that distinguishes positive from negative selection. This could not occur under the differential signaling hypothesis, because it proposes that the signals leading to positive and negative selection are qualitatively different. A new approach to testing these hypotheses has opened up with the recent description of antagonist peptides. These are known as antagonist peptides because they inhibit the response of mature T cells to their normal stimulatory, or agonist, peptide. Recognition of antagonist peptides by thymocytes has been shown to induce positive selection, whereas recognition of agonist peptides induces negative selection. Thus the differences between the ways agonist and antagonist peptides interact with the receptor and signal to the cell are likely to be relevant to the issue of how positive selection works. Initially, affinity measurements were carried out at room temperature and revealed very slight differences in affinity for the agonist versus antagonist peptides. However, the affinities were still broadly similar and this parameter failed to capture a more radical difference in the binding of the T-cell receptor that was observed under these conditions. This suggests that in vivo the agonist peptides induce the receptor clustering required for generating the signals that lead to activation (see Section 6-2) and, during thymocyte development, to deletion. The antagonist peptides, in contrast, bind but fail to induce receptor clustering, and therefore deliver a qualitatively different signal; during thymocyte development, recognition of the antagonist peptide delivers a survival signal to the cell. As the agonist and antagonist peptides bind the T-cell receptor with similar affinity, but only the antagonist induces positive selection, the implication is that the signals delivered by the agonist peptides that cause deletion and by the antagonist peptides that mediate positive selection are fundamentally different. This suggests that the differential signaling hypothesis of T-cell selection is correct. These experiments have, however, only been carried out in a single laboratory, so one should keep an open mind until other laboratories produce similar results in other systems. The differences between negative and positive selection may be due to differences in the aggregation of T-cell receptors upon ligand binding. Agonist, antagonist, and null peptides can be identified for a particular T-cell receptor by assaying the responses of mature T-cell clones. Their effects on mature T cells in vivo can be tested in mice made transgenic for this T-cell receptor (left-hand panels). Binding to an agonist peptide (top panels) induces organized aggregation of T-cell receptors on the cell surface and effective signaling through the T-cell receptor.
It is difficult to prevent the disease, because the organism commonly exists in hospitals, particularly in areas adjacent to infected patients. Thus the organism can contaminate an environment for many months and can be a major source of nosocomial outbreaks of C. A histologic section of colon shows an intense inflammatory response, with the characteristic "plaque" (black arrow) overlying the intact intestinal mucosa (white arrow) (hematoxylin and eosin stain). Note the rectangular shape of the rods, the presence of many decolorized rods appearing gram-negative, and the absence of spore and blood cells. Beta toxin is responsible for intestinal stasis, loss of mucosa with formation of necrotic lesions, and progression to necrotizing enteritis. Epsilon toxin, a protoxin, is activated by trypsin and increases the vascular permeability of the gastrointestinal wall. The enterotoxin is produced during the phase transition from vegetative cells to spores and is released in the alkaline environment of the small intestine when the cells undergo the terminal stages of spore formation (sporulation). The released enterotoxin binds to receptors on the brush border membrane of the small intestine epithelium in the ileum (primarily) and jejunum but not duodenum. Insertion of the toxin into the cell membrane leads to altered membrane permeability and loss of fluids and ions. Spores are formed under adverse environmental conditions and can survive for prolonged periods. Strains of types B through E do not survive in soil but colonize the intestinal tracts of animals and occasionally humans. Five days after the injury, the skin became discolored, and bullae and necrosis developed. A serosanguineous exudate and subcutaneous gas were present, but there was no evidence of muscle necrosis. On March 18, 1993, the Cleveland City Health Department received telephone calls from 15 persons who became ill after eating corned beef purchased from one delicatessen. After publicizing the outbreak, 156 persons contacted the Health Department with a similar history. In addition to a history of diarrhea, 88% complained of abdominal cramps and 13% had vomiting, which developed an average of 12 hours after eating the implicated meat. An investigation revealed the delicatessen had purchased 1400 pounds of raw, salt-cured meat, and beginning on March 12, portions of the corned beef were boiled for 3 hours, allowed to cool at room temperature, and then refrigerated. The Health Department recommended that if the meat could not be served immediately after cooking, it should be rapidly cooled in ice and refrigerated. This is a life-threatening disease that illustrates the full virulence potential of histotoxic clostridia. The onset of disease, characterized by intense pain, generally develops within a week after clostridia are introduced into tissue by trauma or surgery. The onset is followed rapidly by extensive muscle necrosis, shock, renal failure, and death, often within 2 days of initial onset. Gas found in the tissue is caused by the metabolic activity of the rapidly dividing bacteria (hence the name gas gangrene). Clostridial food poisoning (Clinical Case 30-2), a relatively common but underappreciated intoxication, is characterized by (1) a short incubation period (8 to 12 hours), (2) a clinical presentation that includes abdominal cramps and watery diarrhea but no fever, nausea, or vomiting, and (3) a clinical course lasting less than 24 hours. Necrotizing enteritis (also called enteritis necroticans or pig-bel) is a rare necrotizing process in the jejunum characterized by acute abdominal pain, vomiting, bloody diarrhea, ulceration of the small intestine, and perforation of the intestinal wall, leading to peritonitis and shock. Necrotizing enteritis is most common in Papua New Guinea, with sporadic cases reported from other countries.
As explained in Section 7-15, all the T cells developing in such a mouse will express the transgenic receptor. Similar results can be obtained in thymic organ culture with T cells from both normal and transgenic mice, showing that secondary effects caused by the induction of cytokines or corticosteroids due to simultaneous activation of peripheral T cells in vivo cannot account for this cell death of immature thymocytes. If the mice are injected with the peptide that is recognized by the transgenic T-cell receptor, then massive cell death occurs in the thymus, as shown by the increased numbers of apoptotic cells in the right-hand bottom panel. The deletion of developing T cells that recognize self peptides synthesized naturally in the thymus has also been demonstrated experimentally. The negative selection of such thymocytes was observed in mice made transgenic for rearranged genes encoding T-cell receptors specific for self peptides expressed only in male mice. By contrast, in female mice, which lack the male-specific peptide, the transgenic T cells mature normally. This initial observation has been confirmed using T-cell receptor transgenes that recognize other antigens, with similar results. Not all self proteins are expressed in the thymus, however, and those that appear in other tissues, or are expressed at different stages in development, such as after puberty, will encounter mature T cells with the potential to respond to them. However, there are mechanisms that prevent mature T cells from responding to such antigens, and these will be discussed in Chapter 13, when we consider the problem of autoimmune responses and their avoidance. Negative selection is driven most efficiently by bone marrow-derived antigen-presenting cells. These are professional antigen-presenting cell types that also activate mature T cells in peripheral lymphoid tissues. The self antigens presented by these cells are therefore the most important source of potential autoimmune responses, and T cells responding to such self peptides must be eliminated in the thymus. Experiments using bone marrow chimeric mice have shown clearly the role of thymic macrophages and dendritic cells in negative selection. The dendritic cells and macrophages are therefore assumed to have a crucial role in negative selection. In addition, both the thymocytes themselves and thymic epithelial cells also have the ability to cause the deletion of self-reactive cells. Such reactions may normally be of secondary significance compared to the dominant role of bone marrow-derived cells in negative selection. Endogenous superantigens mediate negative selection of T-cell receptors derived from particular V gene segments. It is virtually impossible to demonstrate directly the negative selection of T cells specific for any particular self antigen in the normal thymus because such T cells will be too rare to detect. There is, however, one case in which negative selection can be seen on a large scale in normal mice and the point at which it occurs in T-cell development can be identified. In the most striking examples, T cells expressing receptors encoded by particular V gene segments are virtually eliminated in the affected mouse strains. This occurs as the consequence of the interaction of immature thymocytes with endogenous superantigens present in those strains. Mice that carry these endogenous superantigens are said to be Mls+, and a series of Mls antigens (Mls-1a, Mls-1b. In Mls+ strains, T cells bearing V regions to which the Mls proteins bind, die by apoptosis during intrathymic maturation. Thus, the expression of endogenous superantigens in mice has a profound impact on the T-cell receptor repertoire. This sort of deletion has not yet been seen in any other species, including humans, despite the presence of endogenous retroviral sequences in many mammals. In mice that express the superantigen and thus are tolerant to it, cells expressing receptors responsive to superantigens are found among the double-positive thymocytes and are abundant in thymic cortex. This suggests that superantigens might delete relatively mature T cells as they migrate out of the cortex into the medulla, where a particularly dense network of dendritic cells marks the cortico-medullary junction.
Such a directed mechanism is supported by the observation that individual B cells frequently undergo switching to the same C gene on both chromosomes, even though the antibody heavy chain is only being expressed from one of the chromosomes. Thus, helper T cells regulate both the production of antibody by B cells and the isotype that determines the effector function of the antibody. Antigen-binding B cells are trapped in the T-cell zone of secondary lymphoid tissues and are activated by encounter with armed helper T cells. One of the most puzzling features of the antibody response is how an antigenspecific B cell manages to encounter a helper T cell with an appropriate antigen specificity. This question arises because the frequency of naive lymphocytes specific for any given antigen is estimated to be between 1 in 10,000 and 1 in 1,000,000. Thus, the chance of an encounter between a T lymphocyte and a B lymphocyte that recognize the same antigen should be between 1 in 108 and 1 in 1012. Achieving such an encounter is a far more difficult challenge than getting effector T cells activated, because, in the latter case, only one of the two cells involved has specific receptors. Moreover, T cells and B cells mostly occupy quite distinct zones in peripheral lymphoid tissue. As in naive T-cell activation (see Chapter 8), the answer seems to lie in the antigen-specific trapping of migrating lymphocytes. The medulla consists of strings of macro-phages and antibody-secreting plasma cells known as the medullary cords. Antigen-specific T cells remain in the T-cell zone provided that they encounter antigen on the surface of a antigen-presenting cell such as a dendritic cell. B cells normally move rapidly through the T-cell zone, unless they bind specific antigen, in which case they are trapped before leaving the T-cell zone and thus can interact with antigen-specific armed helper T cells. This interaction gives rise to a primary focus of B cells and T cells near the border between B-cell and T-cell zones. When an antigen is introduced into an animal, it is captured and processed by professional antigen-presenting cells, especially the dendritic cells that migrate from the tissues into the T-cell zones of local lymph nodes. Recirculating naive T cells pass by such cells continuously and those rare T cells whose receptors bind peptides derived from the antigen are trapped very efficiently. This trapping clearly involves the specific antigen receptor on the T cell, although it is stabilized by the activation of adhesion molecules and chemokines as we learned in Sections 8-3 and 84. Ingenious experiments using mice transgenic for rearranged immunoglobulin genes show that, in the presence of the appropriate antigen, B cells with antigen-specific receptors are also trapped in the T-cell zones of lymphoid tissue by a similar mechanism. Trapping of B cells in the T-cell zones provides an elegant solution to the problem posed at the beginning of this section. T cells are themselves trapped and activated to helper status in the T-cell zones, and when B cells migrate into lymphoid tissue through high endothelial venules they first enter these same T-cell zones. Most of the B cells move quickly through the T-cell zone into the B-cell zone (the primary follicle), but those B cells that have bound antigen are trapped. Thus, antigen-binding B cells are selectively trapped in precisely the correct location to maximize the chance of encountering a helper T cell that can activate them. Interaction with armed helper T cells activates the B cell to establish a primary focus of clonal expansion. Here, at the border between T-cell and B-cell zones, both types of lymphocyte will proliferate for several days to constitute the first phase of the primary humoral immune response. However, some of the proliferating B cells differentiate into antibodysynthesizing plasma cells and migrate to the red pulp of the spleen or the medullary cords of the lymph node. The differentiation of a B cell into a plasma cell is accompanied by many morphological changes that reflect its commitment to the production of large amounts of secreted antibody. Plasma cells have abundant cytoplasm dominated by multiple layers of rough endoplasmic reticulum. The nucleus shows a characteristic pattern of peripheral chromatin condensation, a prominent perinuclear Golgi apparatus is visible, and the cisternae of the endoplasmic reticulum are rich in immunoglobulin, which makes up 10 20% of all the protein synthesized. Surface immunoglobulin is still expressed on plasma cells at low levels, and recent evidence suggests that the survival of plasma cells may be determined in part by their ability to continue to bind antigen. Some survive for only days to a few weeks after their final differentiation, whereas others are very long-lived and account for the persistence of antibody responses. Plasma cells secrete antibody at a high rate but can no longer respond to antigen or helper T cells. They can take up antigen and present it to helper T cells, which then induce the B cells to proliferate, switch isotype, and undergo somatic hypermutation; however, B cells do not secrete significant amounts of antibody. Plasma cells have also lost the ability to change isotype or to undergo further somatic hypermutation.
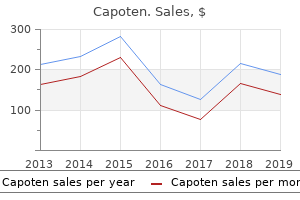
Fumitory. Capoten.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96460

Additionally, PorB with other adhesins facilitates the bacterial invasion into epithelial cells. Finally, expression of some PorB antigens makes the bacteria resistant to complementmediated serum killing. Opa proteins (opacity proteins) are a family of membrane proteins that mediate intimate binding to epithelial and phagocytic cells and are important for cell-to-cell signaling. Opaque colonies are recovered most commonly in patients with localized disease. The third group of proteins in the outer membrane is the highly conserved Rmp proteins (reduction-modifiable proteins). These proteins stimulate antibodies that interfere with the serum bactericidal activity against pathogenic neisseriae. These pathogenic neisseriae are able to compete with their human hosts for iron by binding host cell transferrin to specific bacterial surface receptors. The specificity of this binding for human transferrin is likely the reason these bacteria are strict human pathogens. The presence of this receptor is fundamentally different from most bacteria that synthesize siderophores to scavenge iron. The gonococci also have a variety of additional surface receptors for other host iron complexes, such as lactoferrin and hemoglobin. Table 23-2 Virulence Factors in Neisseria gonorrhoeae Virulence Factor Biological Effect Pilin Protein that mediates initial attachment to nonciliated human cells. Experiments with nasopharyngeal tissue organ cultures have shown that meningococci attach selectively to specific receptors on nonciliated columnar cells of the nasopharynx. Presence of the capsule interferes with epithelial cell attachment, so synthesis is down-regulated before attachment. Following attachment, meningococci are able to multiply, forming large aggregates of bacteria anchored to the host cells. Within a few hours of attachment, the pili undergo posttranslational modification, leading to destabilization of the aggregates. This results in the enhanced ability of the bacteria to both penetrate into the host cells and release into the airways, and thus person-to-person spread is potentially increased. Meningococcal disease occurs in patients who lack specific antibodies directed against the polysaccharide capsule and other expressed bacterial antigens. Infants are initially afforded protection by the passive transfer of maternal antibodies. When the infant has reached age 6 months, however, this protective immunity has waned, a finding consistent with the observation that the incidence of disease is greatest in children younger than 2 years. Patients with deficiencies in C5, C6, C7, or C8 of the complement system are estimated to be at a 6000-fold greater risk for meningococcal disease. Epidemiology Gonorrhea occurs naturally only in humans; it has no other known reservoir. It is second only to chlamydia as the most commonly reported sexually transmitted disease in the United States. Infection rates are the same in males and females, are disproportionately higher in blacks than in Hispanic Americans and whites, and are highest in the southeastern United States. The incidence of disease generally declined after 1978, but the decrease slowed around 1996, and gonococcal infections have increased since 2010. However, even this large number is an underestimation of the true incidence of disease, because diagnosis and reporting of infections are incomplete. Public health officials believe that at least half of new infections are not reported. Women have a 50% risk of acquiring the infection as the result of a single exposure to an infected man, whereas men have a risk of approximately 20% as the result of a single exposure to an infected woman. The risk of infection rises as the person has more sexual encounters with infected partners.
These proteins bind to C3b and C4b on the cell surface, and also mediate protection against complement through the third mechanism, which is to augment the dissociation of C4b,2b and C3b,Bb convertases that have already formed. In addition to the mechanisms for preventing C3 convertase formation and C4 and C3 deposition on cell membranes, there are also inhibitory mechanisms that prevent the inappropriate insertion of the membrane-attack complex into membranes. We saw in Section 2-13 that the membrane-attack complex polymerizes onto C5b molecules released from C5 convertase. This complex mainly inserts into cell membranes adjacent to the site of the C5 convertase, that is, close to the site of complement activation on a pathogen. However, some newly formed membrane-attack complexes may diffuse from the site of complement activation and insert into adjacent host cell membranes. Several plasma proteins bind to the C5b,6,7 complex and thereby inhibit its random insertion into cell membranes. The most important is probably C8 itself, when it binds to C5b,6,7 in the fluid phase. This causes the disease paroxysmal nocturnal hemoglobinuria, which is characterized by episodes of intravascular red blood cell lysis by complement. The complement system is one of the major mechanisms by which pathogen recognition is converted into an effective host defense against initial infection. Complement is a system of plasma proteins that can be activated directly by pathogens or indirectly by pathogen-bound antibody, leading to a cascade of reactions that occurs on the surface of pathogens and generates active components with various effector functions. All three pathways can be initiated independently of antibody as part of innate immunity. The early events in all pathways consist of a sequence of cleavage reactions in which the larger cleavage product binds covalently to the pathogen surface and contributes to the activation of the next component. The pathways converge with the formation of a C3 convertase enzyme, which cleaves C3 to produce the active complement component C3b. The binding of large numbers of C3b molecules to the pathogen is the central event in complement activation. Bound complement components, especially bound C3b and its inactive fragments, are recognized by specific complement receptors on phagocytic cells, which engulf pathogens opsonized by C3b and its inactive fragments. The small cleavage fragments of C3, C4, and especially C5, recruit phagocytes to sites of infection and activate them by binding to specific trimeric G protein-coupled receptors. Together, these activities promote the uptake and destruction of pathogens by phagocytes. The molecules of C3b that bind the C3 convertase itself initiate the late events, binding C5 to make it susceptible to cleavage by C2b or Bb. The larger C5b fragment triggers the assembly of a membrane-attack complex, which can result in the lysis of certain pathogens. The activity of complement components is modulated by a system of regulatory proteins that prevent tissue damage as a result of inadvertent binding of activated complement components to host cells or spontaneous activation of complement components in plasma. Although the innate immune system lacks the specificity of adaptive immunity, it can distinguish nonself from self. We have already seen, in outline, how this is achieved in the complement system and in the response of macrophages to pathogens. In this part of the chapter we will look more closely at the receptors that activate the innate immune response, both those that recognize pathogens directly and those that signal for a cellular response. Proteins that recognize features common to many pathogens occur as secreted molecules and as receptors on cells of the innate immune system. Their general characteristics are contrasted with the antigen-specific receptors of adaptive immunity in. Unlike the receptors that mediate adaptive immunity, the receptors of the innate immune system are typically not clonally distributed; a given set of receptors will be present on all the cells of the same cell type. The binding of pathogens by these receptors gives rise to very rapid responses, which are put into effect without the delay imposed by the clonal expansion of cells needed in the adaptive immune response. The characteristics of receptors of the innate and adaptive immune systems are compared. The innate immune system uses receptors that are encoded by intact genes inherited through the germline, whereas the adaptive immune system uses antigen receptors encoded by genes that are assembled from individual gene segments during lymphocyte development, a process that leads to each individual cell expressing a receptor of unique specificity. As a result, receptors of the innate immune system are deployed nonclonally, whereas the antigen receptors of the adaptive immune system are clonally distributed on individual lymphocytes.
This phase of gene rearrangement lasts for 3 or 4 days in the mouse and only ceases when positive selection occurs as a consequence of receptor engagement, or when the cell dies. Thus, in the strict sense, T-cell receptor -chain genes are not subject to allelic exclusion (see Section 7-10). The regulation of -chain gene rearrangement by positive selection therefore ensures that each T cell has only a single functional specificity, even if two different chains are expressed. Thus, the phase of -chain gene rearrangement marks an important change in the forces shaping the destiny of the T cell. As lymphocytes differentiate from primitive stem cells, they proceed through stages that are marked by the sequential rearrangement of the antigen-receptor gene segments at the different genetic loci. As each complete receptor-chain gene is generated, the protein it encodes is expressed as part of a receptor, and this signals the developing cell to progress to the next developmental step. If rearrangement is successful and a pre-B-cell receptor is made, heavy-chain gene rearrangement ceases and the resulting pre-B cells proliferate, followed by the start of rearrangement at a light-chain locus. If the initial light-chain gene rearrangement is productive, a complete immunoglobulin B-cell receptor is formed, gene rearrangement ceases, and B-cell development proceeds. If it is not, light-chain gene rearrangement continues until either a new productive rearrangement is made or all available J regions have been used up. In developing T cells, receptor loci rearrange according to a defined program similar to that in B cells. There is, however, the added complication that individual precursor T cells can follow one of two distinct lines of development. Rearrangement at these loci leads to cells bearing either: T-cell receptors or: T-cell receptors. Early in ontogeny,: T cells predominate, but from birth onward more than 90% of thymocytes express T-cell receptors encoded by rearranged and genes. In developing thymocytes, the, and loci rearrange first, and start rearranging virtually simultaneously. Productive rearrangements of both a and a gene may lead to the production of a functional: T-cell receptor and the development of a: T cell. In these cells the generation of a -chain gene before both and have rearranged leads to the expression of a functional chain and a pre-T-cell receptor. Thus gene rearrangement follows an ordered sequence in both B and T lineages to produce immature lymphocytes that each bear antigen receptors of a single specificity on their surface. These antigen receptors can now be tested for their antigen-recognition properties and the cells selected accordingly; these selection processes are described in the next part of this chapter. We have followed the development of a lymphocyte from a committed precursor to the point at which a complete antigen receptor is expressed on the cell surface. Development up to this point has been focused on testing for productive gene rearrangements and multiplying those cells that are successful, while at the same time controlling the rearrangement process so that cells express only one receptor. Now the fate of the immature lymphocyte will be determined by the specificity of its antigen receptor. Most obviously, lymphocytes with strongly self-reactive receptors should be eliminated to prevent autoimmune reactions; this negative selection is one of the ways in which the immune system is made self-tolerant. In addition, given the incredible diversity of receptors that the rearrangement process can generate, it is important that those lymphocytes that mature are likely to be useful in recognizing and responding to foreign antigens, especially as an individual can only express a small fraction of the total possible receptor repertoire in his or her lifetime. Indeed, certainly for T cells and probably for B cells, a process of positive selection identifies and preserves lymphocytes that are likely to be able to respond to foreign antigens; those that do not pass this test die by neglect. In this part of the chapter we will describe how the processes of positive and negative selection shape the mature lymphocyte repertoire. Immature B cells that bind self antigens undergo further receptor rearrangement, or die, or are inactivated. Once an immature B cell expresses IgM on its surface (sIgM), its fate is guided by the nature of the signals it receives through its antigen receptor. This was first demonstrated by experiments in which antigen receptors on immature B cells were experimentally stimulated in vivo using anti- chain antibodies (see Appendix I, Section A-10); the outcome was elimination of the immature B cells. More recent experiments using mice expressing B-cell receptor transgenes have confirmed these earlier findings, but have also shown that immediate elimination is not the only possible outcome of binding to a self antigen. Instead, there are four possible fates for self-reactive immature B cells, depending on the nature of the ligand to which they are capable of binding.
It is responsible for infections in debilitated patients with impaired host defense mechanisms. Hospital infections with this organism have been traced to contaminated intravenous catheters, disinfectant solutions, mechanical ventilation equipment, and ice machines. Antimicrobial therapy is complicated because the organism is resistant to many commonly used drugs. They are ubiquitous saprophytes, recovered in nature and in the hospital and able to survive on both moist surfaces, such as mechanical ventilation equipment, and on dry surfaces, such as human skin (the latter feature is unusual for gram-negative rods). These bacteria are also part of the normal oropharyngeal flora of a small number of healthy people and can proliferate to large numbers during hospitalization. Acinetobacters are opportunistic pathogens (see Box 27-1) that cause infections in the respiratory tract, urinary tract, and wounds; they also cause septicemia. Patients at risk for Acinetobacter infections are those receiving broadspectrum antibiotics, recovering from surgery, or on respiratory ventilation. Nosocomial wound and pulmonary infections in hospitalized patients have become a significant problem because many of the infections are caused by strains resistant to most antibiotics, including the carbapenems. Care must be taken when carbapenems or colistin are selected, because in vitro tests may not reliably detect heteroresistant strains. E1 Case Study and Questions A 63-year-old man has been hospitalized for 21 days for the management of newly diagnosed leukemia. Three days after he entered the hospital, a urinary tract infection with Escherichia coli developed. What virulence factors possessed by the organism make it a particularly serious pathogen The capsule also can function as an adhesion factor and interfere with phagocytosis. A variety of other enzymes (exoenzymes S and T, elastases, alkaline protease, phospholipase C) contribute to the tissue damage characteristic of Pseudomonas infections. Mutation of porin proteins can interfere with the penetration of many classes of antibiotics through the outer membrane and into the bacterial cell. Pseudomonas also produces a variety of -lactamases that can inactivate -lactam antibiotics, including carbapenems such as imipenem and meropenem. Resistance to many antibiotics has been reported, so effective therapy requires in vitro susceptibility tests. Empirical therapy for serious infections should use a combination of a broad-spectrum -lactam. Although most isolates are resistant to penicillins, the bacteria are uniformly susceptible to other antibiotics. Examination of the plates after 42 hours revealed the presence of flat, nonhemolytic, mucoid colonies that were subsequently identified as Campylobacter jejuni. Campylobacter and Helicobacter are now widely recognized as significant human pathogens; however, they were only discovered in the last 20 to 30 years. Campylobacter is thin, at the resolving power of light microscopy, and is not typically observed in Gramstained specimens. Helicobacter is also difficult to grow, requiring enriched media, a microaerophilic atmosphere, and prolonged incubation. The bacteria are actively motile and rapidly penetrate through the gastric mucus and adhere to gastric epithelial cells, followed by penetration into the cells.
Evidence that the hinge region plays a role in maintaining serum levels of the murine IgG1 molecule Mol. Rapid and reliable cloning of antibody variable regions and generation of recombinant single-chain antibody fragments Tissue Antigens 1996. Kabat Database and its applications: 30 years after the first variability plot Nucleic Acids Res. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity J. Antibody-antigen interactions: new structures and new conformational changes Curr. Anatomy of an antibody molecule: structure, kinetics, thermodynamics and mutational studies of the antilysozyme antibody D1 Immunol. Antigen binding forces of individually addressed single-chain Fv antibody molecules Proc. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme Immunity 1998. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity Immunity 1999. The Generation of Lymphocyte Antigen Receptors Introduction to Chapter 4 the generation of diversity in immunoglobulins T-cell receptor gene rearrangement Structural variation in immunoglobulin constant regions Summary to Chapter 4 References to Chapter 4 Introduction to Chapter 4 Lymphocyte antigen receptors, in the form of immunoglobulins on B cells and T-cell receptors on T cells, are the means by which lymphocytes sense the presence of antigens in their environment. The receptors produced by each lymphocyte have a unique antigen specificity, which is determined by the structure of their antigen-binding site, as described in Chapter 3. Because each person possesses billions of lymphocytes, these cells collectively provide the individual with the ability to respond to a great variety of antigens. The wide range of antigen specificities in the antigen receptor repertoire is due to variation in the amino acid sequence at the antigen-binding site, which is made up from the variable (V) regions of the receptor protein chains. In each chain the V region is linked to an invariant constant (C) region, which provides effector or signaling functions. Given the importance of a diverse repertoire of lymphocyte receptors in the defense against infection, it is not surprising that a complex and elegant genetic mechanism has evolved for generating these highly variable proteins. Each receptor chain variant cannot be encoded in full in the genome, as this would require more genes for antigen receptors than there are genes in the entire genome. Instead, we will see that the V regions of the receptor chains are encoded in several pieces so-called gene segments. The selection of a gene segment of each type during gene rearrangement occurs at random, and the large number of possible different combinations accounts for much of the diversity of the receptor repertoire. In the first two parts of this chapter we will describe the gene rearrangement mechanism that generates the V regions of immunoglobulin and T-cell receptor genes. The basic mechanism is common to both B cells and T cells, and involves many if not all of the same enzymes. We will describe the details of the enzymology of this recombination process, the evolution of which was probably critical to the evolution of the vertebrate adaptive immune system. In B cells, but not T cells, the rearranged V region undergoes additional modification, known as somatic hypermutation. This does not occur until after B cells encounter and become activated by antigen. In these cells, the V regions of the assembled immunoglobulin genes undergo a high rate of point mutation that creates additional diversity within the expanding clone of B cells responding to antigen. In the third part of the chapter we consider the limited, but functionally important, diversity of immunoglobulin C regions. The C regions of T-cell receptors do not show such diversity as they function only as part of a membranebound antigen receptor. Their role is to anchor and support the V regions at the cell surface as well as linking the binding of antigen by the V regions to the receptor-associated intracellular signaling complex. The C regions of immunoglobulins also serve these functions but in addition the C regions of the heavy chain are responsible for the effector functions of the secreted immunoglobulins, or antibodies, made by activated B cells. This enables the different heavy-chain C regions, each with a different function, to be represented among antibodies of the same antigen specificity.
References: